Deficiency of the Interleukin-1 Receptor Antagonist (DIRA)
Rare autoinflammatory disorder causing severe pustulosis, osteomyelitis, and systemic inflammation in newborns, treatable with IL-1 blockade.
Deficiency of the interleukin-1 receptor antagonist (DIRA) is a rare, autosomal recessive autoinflammatory disorder caused by mutations in the IL1RN gene, leading to unopposed interleukin-1 (IL-1) signaling and severe systemic inflammation starting at birth.
What is DIRA?
DIRA, also known as interleukin-1 receptor antagonist deficiency, manifests in newborns with a characteristic triad of generalized pustulosis, multifocal aseptic osteomyelitis, and markedly elevated acute-phase reactants like C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Without treatment, it progresses to multiorgan failure and death in early childhood, but interleukin-1 blockade therapy dramatically improves outcomes.
The condition arises from loss-of-function mutations in IL1RN, which encodes the IL-1 receptor antagonist (IL-1Ra), a natural inhibitor of pro-inflammatory IL-1α and IL-1β cytokines. Absent functional IL-1Ra, excessive IL-1 activity drives uncontrolled innate immune responses, primarily affecting skin, bone, and periarticular structures.
Who gets DIRA?
DIRA is extremely rare, with fewer than 20 cases reported worldwide, primarily in consanguineous families of Puerto Rican, Dutch, or Middle Eastern descent due to founder mutations. It affects both genders equally as an autosomal recessive trait, presenting exclusively in infancy—typically at birth or within the first weeks of life.
Neonates from unaffected parents who are heterozygous carriers are at risk if both inherit the mutated allele. Prenatal onset may occur, evidenced by fetal distress or radiographic bone changes detectable in utero.
What causes DIRA?
Pathogenic biallelic mutations in IL1RN on chromosome 2q14.1 abolish IL-1Ra protein production or function, removing inhibition of IL-1 receptors (IL-1R1). Common variants include nonsense mutations (e.g., c.21G>A, p.Trp7Ter in Puerto Rican cases) and deletions leading to frameshifts.
This genetic defect results in hyperactive IL-1 signaling via NF-κB pathways, promoting neutrophil recruitment, cytokine storms, and sterile inflammation in skin (pustules), bone (periostitis/osteomyelitis), and periosteum. Variable mutation effects explain phenotypic heterogeneity—some patients show predominant skin disease, others severe skeletal involvement.
What are the clinical features of DIRA?
Distinctive features emerge perinatally without fever, including:
- Skin findings: Pustular eruption (discrete crops or generalized resembling pustular psoriasis), erythematous plaques, desquamation, ichthyosis-like scaling, nail dystrophy.
- Mucosal involvement: Stomatitis, oral ulcers, conjunctivitis.
- Musculoskeletal: Painful joint swelling, contractures, aseptic multifocal osteomyelitis, periostitis, periosteal elevation.
- Systemic: Hepatosplenomegaly, failure to thrive, respiratory distress, interstitial lung disease.
- Rare: Vasculitis (cerebral, cutaneous), thrombosis, hemophagocytic lymphohistiocytosis.
Infants appear critically ill with edema, widespread pustules, and bony tenderness. Bone involvement universally causes movement pain; radiographs show pathognomonic changes like rib-end ballooning.
How is DIRA diagnosed?
Diagnosis combines clinical, laboratory, imaging, histopathology, and genetic confirmation:
| Modality | Findings |
|---|---|
| Blood tests | Leukocytosis, thrombocytosis, anemia, elevated ESR/CRP |
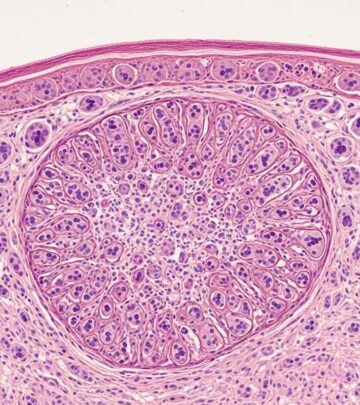
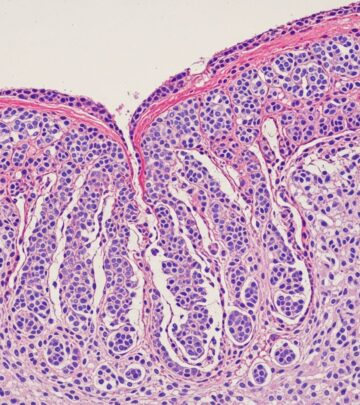
| Skin biopsy | Neutrophilic pustules, acanthosis, hyperkeratosis, dermal infiltrates, vasculitis |
| Bone imaging | Periosteal reaction, osteolysis, rib expansion, heterotopic ossification |
| Bone biopsy | Suppurative osteomyelitis without organisms, fibrosis, sclerosis |
| Genetics | Biallelic IL1RN mutations (NGS/sanger sequencing) |
Differential includes chronic infantile neurological cutaneous articular syndrome (CINCA), Majeed syndrome, and infection; failure of antibiotics and pathognomonic radiology/genetics distinguish DIRA.
What is the treatment of DIRA?
First-line therapy is recombinant IL-1Ra (anakinra, 1-8 mg/kg/day subcutaneously), providing immediate remission of inflammation, pustules, and bone pain. Anakinra reconstitutes IL-1 blockade, normalizing acute-phase reactants within days.
Corticosteroids offer temporary control but cause growth suppression and osteoporosis; reserved for bridging. Anakinra side effects include injection-site reactions (resolving in weeks), rare anaphylaxis (managed by desensitization), infections, neutropenia.
Long-term anakinra sustains remission; weaning risks relapse. Canakinumab or rilonacept (IL-1 blockers) are alternatives if anakinra-intolerant. Multidisciplinary care addresses complications like respiratory failure or thrombosis.
What is the outcome for DIRA?
Untreated DIRA causes fatal multiorgan failure by age 2-3 years from pulmonary fibrosis, sepsis-like crises, or bone deformities. Anakinra yields rapid, sustained remission in all reported cases, with catch-up growth, rash resolution, and halted bone progression—potentially curative if started early.
Some residual skeletal deformities persist from delays in diagnosis. Lifelong therapy appears necessary; fertility/pregnancy data limited. Prognosis excels with prompt genetic diagnosis and IL-1 inhibition.
Clinical images of DIRA
Images typically depict neonates with diffuse pustulosis, crusted plaques on trunk/extremities, oral ulcers, swollen tender joints, and radiographs showing periosteal cloaking of long bones, lytic lesions, and expanded anterior rib ends.
Frequently asked questions about DIRA
Is DIRA curable?
Not genetically curable, but anakinra provides complete clinical and inflammatory control, preventing progression and enabling normal development.
How soon after birth does DIRA appear?
Symptoms onset at birth or within days, with pustules, bone pain, and lab abnormalities evident neonatally.
Can DIRA be mistaken for infection?
Yes, aseptic osteomyelitis mimics bacterial disease; antibiotics fail, and biopsies lack organisms.
What if a child with pustulosis and bone pain tests negative for IL1RN mutations?
Consider related IL-1 mediated diseases like NOMID/CINCA (NLRP3 mutations) or Majeed syndrome.
Does DIRA cause fever?
No, fever is atypical despite severe inflammation; this differentiates from other autoinflammations.
References
- Deficiency of the interleukin-1–receptor antagonist — Wikipedia. 2023. https://en.wikipedia.org/wiki/Deficiency_of_the_interleukin-1–receptor_antagonist
- Deficiency of Interleukin-1 Receptor Antagonist: New Genetic… — PMC (NIH). 2023-08-21. https://pmc.ncbi.nlm.nih.gov/articles/PMC10421680/
- Deficiency of Interleukin-1 Receptor Antagonist (DIRA) — PMC (NIH). 2021. https://pmc.ncbi.nlm.nih.gov/articles/PMC8420971/
- Deficiency of the interleukin-1 receptor antagonist (DIRA) — DermNet NZ. 2024. https://dermnetnz.org/topics/deficiency-of-the-interleukin-1-receptor-antagonist-dira
- Deficiency of Il-1 Receptor Antagonist (Il-1 RA): DIRA — NOMID Alliance. 2023. https://www.nomidalliance.org/dira.php
- Understanding DIRA Autoinflammatory Disease — Kiniksa Pharmaceuticals. 2024. https://www.kiniksa.com/dira
Read full bio of Sneha Tete