EBV-Associated Lymphoproliferative Disorders
Comprehensive overview of Epstein-Barr virus-linked lymphoproliferative disorders, from benign proliferations to aggressive lymphomas.
Epstein–Barr virus-associated lymphoproliferative disorders
Epstein–Barr virus (EBV), a member of the herpesvirus family, infects over 90% of the global adult population and primarily targets B lymphocytes. While most infections are asymptomatic or cause self-limited infectious mononucleosis, EBV can lead to a spectrum of lymphoproliferative disorders (LPDs), particularly in immunocompromised individuals. These disorders range from benign polyclonal expansions to aggressive monoclonal lymphomas. Predisposing factors include iatrogenic immunosuppression (e.g., post-transplant), congenital immunodeficiencies, HIV infection, and age-related immunosenescence.
In the skin, EBV-associated LPDs manifest as EBV-positive mucocutaneous ulcer (EBV-MCU), lymphomatoid granulomatosis (LYG), and primary cutaneous plasmablastic lymphoma (PBL). Accurate diagnosis relies on histopathology, immunohistochemistry, and EBV detection via in situ hybridization for EBV-encoded small RNAs (EBER). This article details their clinical features, histopathology, diagnosis, and management.
What is the Epstein–Barr virus?
EBV, also known as human herpesvirus 4 (HHV-4), is transmitted via saliva and establishes lifelong latency in B cells. Primary infection often presents as infectious mononucleosis with fever, pharyngitis, lymphadenopathy, and atypical lymphocytosis, resolving within 2–6 weeks. In immunocompetent hosts, EBV-specific cytotoxic T cells control proliferation; however, immunosuppression allows uncontrolled expansion of EBV-infected B cells, leading to LPDs.
EBV latency programs include Latency I (EBNA1 only, e.g., Burkitt lymphoma), Latency II (EBNA1, LMP1, LMP2; e.g., Hodgkin lymphoma), and Latency III (full expression; post-transplant LPDs). Skin manifestations typically show Latency II patterns.
Who gets Epstein–Barr virus-associated lymphoproliferative disorders?
EBV-LPDs predominantly affect immunocompromised patients:
- Post-transplant recipients: Solid organ or hematopoietic stem cell transplant patients on immunosuppressants.
- HIV/AIDS patients: Especially with low CD4 counts.
- Primary immunodeficiencies: e.g., X-linked lymphoproliferative disease (XLP), common variable immunodeficiency (CVID).
- Iatrogenic immunosuppression: Methotrexate for rheumatologic diseases, age-related immunosenescence.
- Immunocompetent elderly: Rare for EBV-MCU.
Skin involvement is more common in post-transplant settings for PBL and LYG.
What causes Epstein–Barr virus-associated lymphoproliferative disorders?
Pathogenesis involves impaired T-cell surveillance allowing EBV-driven B-cell proliferation. EBV proteins like LMP1 mimic CD40 signaling, promoting survival and proliferation. Clonal selection occurs via somatic hypermutations and genomic instability (e.g., TP53 mutations, MYC amplifications). In skin lesions, local factors like diminished T-cell populations lead to EBV-specific T-cell clones and localized lymphoproliferation.
What are the clinical features of Epstein–Barr virus-associated lymphoproliferative disorders?
Cutaneous presentations vary by subtype:
- EBV-MCU: Solitary, well-circumscribed ulcers on orolabial mucosa, skin, or Waldeyer ring.
- LYG: Multiple erythematous papules, nodules, or plaques on extremities, progressing to ulceration and necrosis.
- Primary cutaneous PBL: Purple nodules, erythematous plaques, or ulcerative lesions, often on legs.
Systemic symptoms (fever, weight loss) are uncommon in isolated cutaneous disease but frequent in disseminated LPDs.
How is Epstein–Barr virus-associated lymphoproliferative disorder diagnosed?
Diagnosis combines clinical, histopathologic, immunophenotypic, and molecular findings:
- Biopsy: Essential for architecture, cytology, and EBER in situ hybridization.
- Immunohistochemistry: CD20, CD79a, PAX5 for B cells; CD30, CD15 variable.
- EBV detection: EBER-ISH confirms EBV association.
- Clonality: PCR for IgH/TCR rearrangements.
- Imaging/staging: PET-CT for extracutaneous disease.
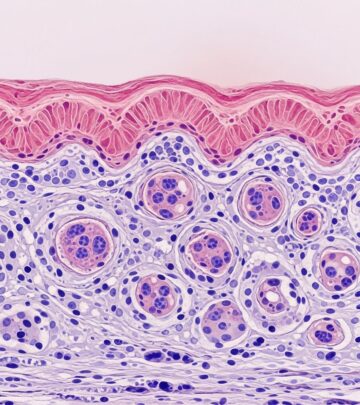
Histopathology of Epstein–Barr virus-associated lymphoproliferative disorders
Key histologic features:
| Disorder | Histology | Immunophenotype |
|---|---|---|
| EBV-MCU | Polymorphic infiltrate with EBV+ B cells, RS-like cells, necrosis; confined to mucosa/skin | CD20+, CD79a+, EBER+; T cells CD3+, CD8+ |
| LYG | Angiocentric/angiodestructive EBV+ B-cell infiltrate (Grade 1-3 based on large cells); necrosis | EBER+ atypical B cells; CD3+ T cells, histiocytes |
| Primary cutaneous PBL | Diffuse sheets of plasmacytoid large cells | CD79a+, MUM1/IRF4+, CD138+/-; CD20 often negative, EBER+ |
EBV+ cells show Latency II pattern.
Differential diagnosis of Epstein–Barr virus-associated lymphoproliferative disorders
- EBV-MCU vs. DLBCL: MCU lacks systemic disease, more polymorphic.
- LYG vs. GPA/WG: EBER distinguishes vasculitic lymphoma from vasculitis.
- PBL vs. Plasmacytoma/Myeloma: PBL EBER+, more aggressive.
- Other: Hydroa vacciniforme-like LPD, peripheral T-cell lymphoma.
EBV-positive mucocutaneous ulcer
Clinical features
EBV-MCU presents as solitary punched-out ulcers (1–2 cm) with rolled borders, commonly on lower lip, tongue, palate, or skin (face, extremities). Painful but indolent; rare dissemination. Predisposing: Immunosuppression, elderly.
Histopathology
Ulcer base shows mixed infiltrate: small lymphocytes, plasma cells, histiocytes, EBV+ large B cells/RS-like cells. Necrosis common; no deep invasion.
Immunohistochemistry
EBV+ cells: CD20+, CD79a+, PAX5+/-, CD30+, EBER+. Background: CD3+, CD8+ T cells, CD68+ histiocytes.
Management
Benign course; options:
- Reduce immunosuppression.
- Rituximab, radiation (local), surgery.
- Observation if asymptomatic.
Prognosis excellent; <5% progress.
Lymphomatoid granulomatosis
Clinical features
Multi-site: Skin (crops of crops of papulonodules, ulcers), lung, kidney, CNS. Skin: Extremities trunk; violaceous papules → necrotic ulcers. Systemic: B symptoms, pulmonary infiltrates.
Histopathology
Grades 1–3: EBV+ large B cells in angiocentric polymorphic infiltrate; necrosis, vascular damage. Grade 3: Monomorphic lymphoma.
Immunohistochemistry
EBER+ B cells (CD20+); rich T-cell (CD3+, CD4+), histiocytic infiltrate.
Management
Grade 1–2: Reduce immunosuppression, rituximab, interferon-alpha. Grade 3: R-CHOP chemotherapy. Poor prognosis for high-grade (5-year survival ~25%).
Primary cutaneous plasmablastic lymphoma
Clinical features
Rare; purple/red nodules, plaques, tumors/ulcers on legs, trunk. Often post-transplant or HIV; immunocompetent possible.
Histopathology
Diffuse proliferation of medium-large plasmacytoid cells; apoptotic bodies.
Immunohistochemistry
CD20 often -; CD79a+, MUM1+, CD138+/-, Ki67 high, EBER+. Aberrant CD13 possible.
Management
Reduce immunosuppression; chemotherapy (CHOP-like), radiation, rituximab, proteasome inhibitors. Guarded prognosis.
Other EBV-associated lymphoproliferative disorders
- EBV+ DLBCL: Monomorphic large B cells, skin rare.
- CAEBV: Chronic fever, organomegaly, high EBV-DNA.
- HLH: Cytopenias, hemophagocytosis, high ferritin.
Frequently asked questions
Q: Can EBV-LPDs occur in immunocompetent patients?
A: Yes, particularly EBV-MCU in elderly; rare for others.
Q: Is EBER staining essential for diagnosis?
A: Yes, confirms EBV association in atypical lymphoid proliferations.
Q: What is the prognosis of cutaneous LYG?
A: Better if skin-limited; systemic disease has poor survival.
Q: How to manage post-transplant EBV-LPD?
A: Reduce immunosuppression first-line; rituximab if persistent.
Q: Does HIV status affect PBL prognosis?
A: EBV+ PBL may have better response in some studies.
References
- Epstein-Barr Virus–Associated Lymphoproliferative Disorders — Front Immunol. 2017-07-25. https://pmc.ncbi.nlm.nih.gov/articles/PMC5525035/
- Epstein–Barr virus-associated lymphoproliferative disorders — DermNet NZ. 2023. https://dermnetnz.org/topics/epsteinbarr-virus-associated-lymphoproliferative-disorders
- EBV-Related Lymphoproliferative Diseases: A Review — Front Immunol. 2024. https://pmc.ncbi.nlm.nih.gov/articles/PMC11178045/
- Epstein-Barr virus-associated lymphoproliferative disorders in immunodeficiency — Ann Lymph. 2020. https://aol.amegroups.org/article/view/7604/html
- How I treat EBV lymphoproliferation — Blood. 2009-11-05. https://ashpublications.org/blood/article/114/19/4002/26734/How-I-treat-EBV-lymphoproliferation
Read full bio of Sneha Tete