Fibrosarcoma Pathology
Comprehensive overview of fibrosarcoma pathology, including clinical features, histopathology, diagnosis, and management strategies.
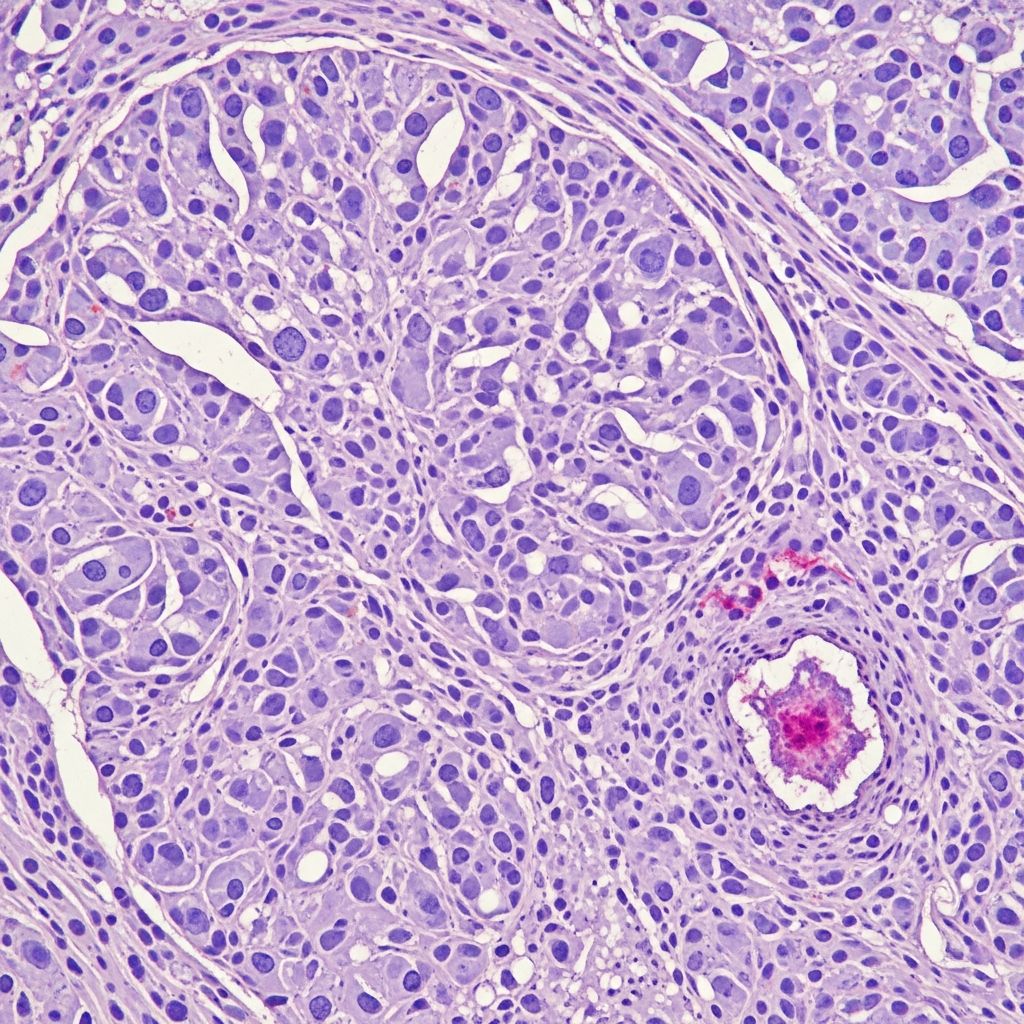
Fibrosarcoma represents a rare and aggressive malignant neoplasm originating from fibroblasts, characterized by the proliferation of spindle-shaped cells with variable collagen production in a fascicular or herringbone pattern. This tumour primarily affects soft tissues but can involve the dermis and subcutis in cutaneous presentations. Understanding its pathology is crucial for accurate diagnosis and management, given its potential for local invasion and metastasis.
Introduction
Fibrosarcoma is classified as a fibroblastic sarcoma, encompassing both conventional adult-type and infantile variants. Adult fibrosarcoma typically arises in deep soft tissues of the extremities, trunk, or head and neck in individuals aged 35–55 years, presenting as a painless, firm mass. In contrast, infantile fibrosarcoma occurs in children under 10 years, often in distal extremities, and exhibits a more favorable prognosis despite high-grade histology. Cutaneous involvement is uncommon but documented, often mimicking other spindle cell tumours. The hallmark genetic event in dermatofibrosarcoma protuberans (DFSP), a related entity sometimes considered in the fibrosarcomatous spectrum, is the t(17;22) translocation resulting in COL1A1-PDGFB fusion, though true fibrosarcoma lacks this.
Clinical Features
Clinically, fibrosarcoma manifests as a slowly enlarging, painless subcutaneous or dermal nodule, often on the trunk, extremities, or head. Early lesions may appear plaque-like or indurated, progressing to protuberant nodules measuring 1–5 cm or larger. Skin changes include erythema, telangiectasia, or ulceration in advanced cases. Pain, tenderness, or neurological symptoms arise with deep invasion. In infantile cases, rapid growth is noted within weeks of birth. Differential clinical considerations include DFSP, leiomyosarcoma, and benign fibrous histiocytoma. Incidence is low, approximately 1–2% of adult soft tissue sarcomas.
- Slow-growing firm nodule or plaque on trunk/extremities
- Age predilection: Adults 35–55 years; infantile <10 years
- Rare cutaneous involvement; deep soft tissue predominant
- Potential for local recurrence and metastasis (lungs, bones)
Histopathology
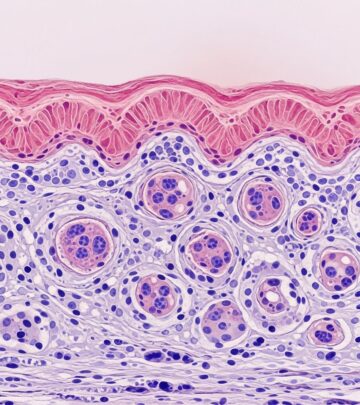
Microscopically, fibrosarcoma displays poorly circumscribed infiltrative growth of uniform spindle cells arranged in long sweeping fascicles with a characteristic herringbone pattern, reminiscent of skeletal muscle. Cells have elongated nuclei with tapered ends, scant cytoplasm, and minimal pleomorphism in low-grade forms. Collagen bands separate fascicles. Mitotic activity varies (5–20/10 HPF), with atypical forms in high-grade tumours. Necrosis is focal; myxoid change or giant cells may occur. Infantile variants show higher cellularity and mitoses but behave indolently. Fibrosarcomatous transformation in DFSP (10–20% cases) features higher-grade herringbone areas with necrosis and increased mitoses (>10/10 HPF).
The tumour permeates dermis and subcutis in a lattice-like pattern, sparing epidermis. Deep extension to fascia/muscle is common in recurrences. Grading relies on mitotic rate, necrosis, and pleomorphism: low-grade (<5 mitoses/10 HPF, no necrosis); high-grade (>10 mitoses/10 HPF, necrosis present).
| Feature | Low-Grade Fibrosarcoma | High-Grade Fibrosarcoma |
|---|---|---|
| Mitotic rate | <5/10 HPF | >10/10 HPF |
| Necrosis | Absent | Focal/extensive |
| Pleomorphism | Mild | Marked |
| Pattern | Fascicular | Herringbone + atypia |
Cytology
Cytological smears reveal dispersed spindle cells with elongated, hyperchromatic nuclei, fine chromatin, and inconspicuous nucleoli. Background contains collagen fibrils. Cellularity is high in infantile cases. Fine-needle aspiration is limited due to spindle morphology overlap with benign lesions; core biopsy preferred for architecture.
Histological Variants
- Conventional (adult-type): Monophasic spindle cells, herringbone pattern, moderate collagen.
- Infantile: Hypercellular, myxoid areas, high mitoses but low metastasis risk.
- Myxofibrosarcoma: Myxoid matrix, curvilinear vessels (overlaps high-grade).
- Giant cell variant: Multinucleated giants, pleomorphic.
- Fibrosarcomatous DFSP: Transitional from storiform DFSP, aggressive foci.
Immunohistochemistry
Fibrosarcoma shows variable
vimentin
positivity (universal fibroblastic marker). Focalactin
ordesmin
in some;CD34
positive in DFSP-like areas but negative in pure fibrosarcoma. Negative forS100
,EMA
,keratins
,h-caldesmon
. Proliferation indexKi67
>20% in high-grade. PDGFB overexpression in DFSP-fibrosarcomatous areas. IHC aids differentiation: e.g., from MPNST (S100+), leiomyosarcoma (smooth muscle actin+).| Marker | Fibrosarcoma | DFSP | Leiomyosarcoma |
|---|---|---|---|
| CD34 | Focal/neg | Diffuse+ | Neg |
| S100 | Neg | Neg | Neg |
| Actin | Focal | Neg | Diffuse+ |
| Ki67 | Variable high | Low | Variable |
Differential Diagnosis
Key mimics include:
- **DFSP:** Storiform pattern, CD34+, COL1A1-PDGFB fusion; less aggressive.
- **Dermatofibroma:** Epidermal hyperplasia, epidermal-dermal interface trapping, factor XIIIa+.
- **Leiomyosarcoma:** Cigar nuclei, actin+, desmin+.
- **Monophasic synovial sarcoma:** SYT-SSX fusion, EMA+.
- **Nodular fasciitis:** Loose storiform, extravasated RBCs, rapid growth.
- **Malignant peripheral nerve sheath tumour:** S100+, wavy nuclei.
Molecular testing (FISH for t(17;22), next-gen sequencing) resolves ambiguities.
Electron Microscopy
Ultrastructurally, fibrosarcoma fibroblasts feature rough ER, Golgi, and sparse collagen fibrils. No specific junctions or melanosomes, distinguishing from neural or myogenic tumours. Rough ER dilation indicates synthetic activity.
Genetics
Adult fibrosarcoma lacks recurrent translocations; complex karyotypes with TP53, RB1 mutations common in high-grade. Infantile type harbours ETV6-NTRK3 fusion (t(12;15)). DFSP-fibrosarcomatous areas retain COL1A1-PDGFB. NGS panels detect these for targeted therapy (e.g., imatinib for PDGFB).
Treatment and Prognosis
Wide local excision (2–3 cm margins to fascia) is standard; Mohs micrographic surgery for cutaneous sites (recurrence <5%). Radiation adjunctive for close margins (recurrence reduction 50%). High-grade/inoperable: neoadjuvant chemo (doxorubicin/ifosfamide), targeted TKIs (imatinib for DFSP). Prognosis: infantile >90% survival; adult low-grade 70–90% 5-year, high-grade 30–50% with metastasis risk (lungs 20–30%). Local recurrence 20–40% without adequate margins.
Frequently Asked Questions
What is the hallmark histological pattern of fibrosarcoma?
The herringbone pattern of intersecting fascicles of spindle cells.
How does fibrosarcoma differ immunohistochemically from DFSP?
Fibrosarcoma is CD34 negative or focal; DFSP diffusely CD34 positive.
Is infantile fibrosarcoma aggressive?
Despite high-grade histology, it has excellent prognosis with >90% survival.
What is the first-line treatment for cutaneous fibrosarcoma?
Wide excision or Mohs surgery with clear margins.
Does fibrosarcoma metastasize?
Yes, high-grade forms to lungs/bones; low-grade rarely.
References
- Dermatofibrosarcoma Protuberans – StatPearls — NCBI Bookshelf. 2023-08-08. https://www.ncbi.nlm.nih.gov/books/NBK513305/
- Dermatofibrosarcoma Protuberans (DFSP): Causes & Treatment — Cleveland Clinic. 2023-11-15. https://my.clevelandclinic.org/health/diseases/24068-dermatofibrosarcoma-protuberans
- Fibrosarcoma – StatPearls — NCBI Bookshelf. 2023-07-17. https://www.ncbi.nlm.nih.gov/books/NBK560759/
- Dermatofibrosarcoma protuberans — DermNet NZ. 2023. https://dermnetnz.org/topics/dermatofibrosarcoma-protuberans
Read full bio of medha deb