Tildrakizumab
Tildrakizumab: A targeted IL-23 inhibitor for effective management of moderate-to-severe plaque psoriasis in adults.
Author: Reviewed by Dermatologists
Tildrakizumab is a biologic medication specifically designed for the treatment of moderate-to-severe plaque psoriasis in adults. Marketed as Ilumya )”, it represents a significant advancement in targeted immunotherapy by selectively inhibiting the interleukin-23 (IL-23) pathway, a key driver of psoriatic inflammation. This article explores its pharmacology, clinical use, efficacy data from pivotal trials, administration guidelines, safety considerations, and monitoring protocols.
What is tildrakizumab?
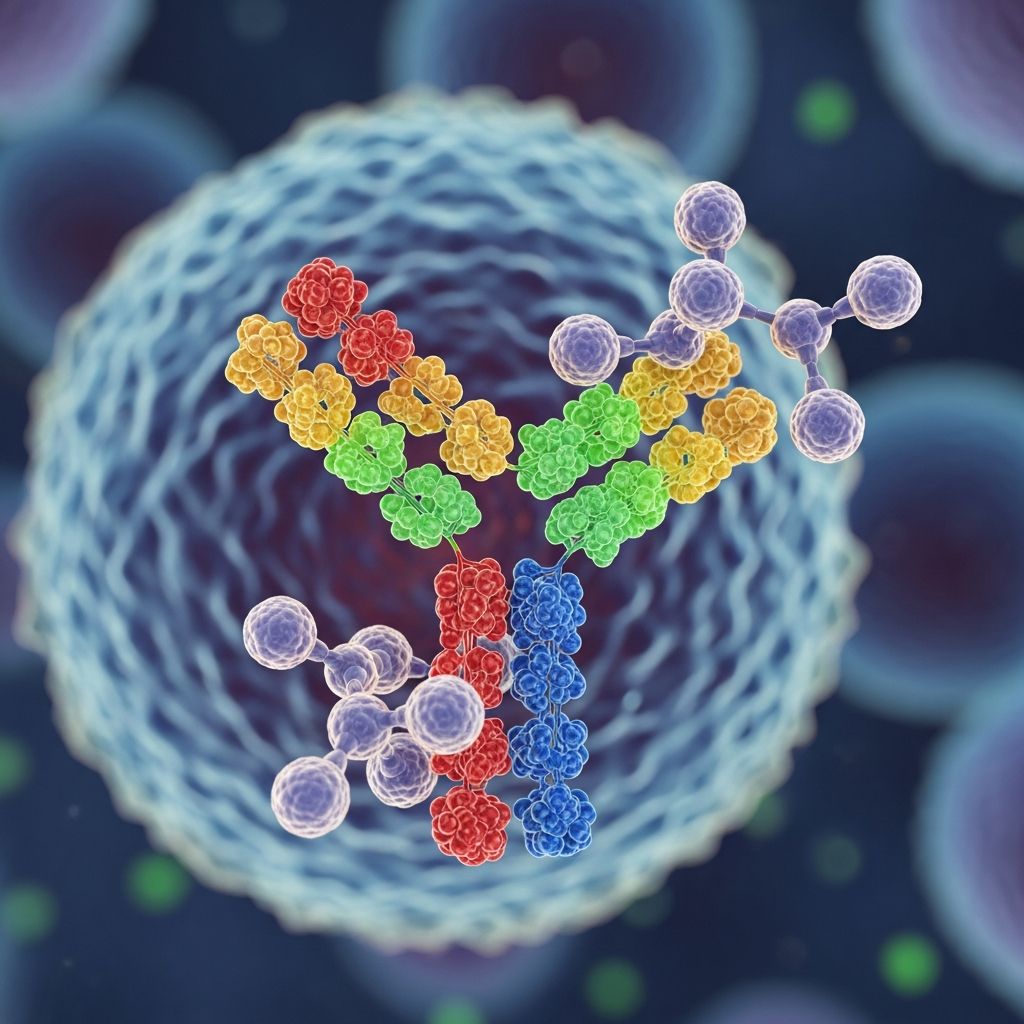
Tildrakizumab-asmn is a humanized immunoglobulin G1 kappa (IgG1) monoclonal antibody engineered to precisely target the p19 subunit of interleukin-23 (IL-23). Unlike broader immunosuppressants, tildrakizumab offers high specificity, minimizing off-target effects while effectively disrupting the inflammatory cascade central to psoriasis pathogenesis.
IL-23 is a pro-inflammatory cytokine composed of p19 and p40 subunits. In psoriasis, elevated IL-23 levels in lesional skin drive Th17 cell differentiation, leading to excessive production of IL-17, IL-22, and other mediators that promote keratinocyte hyperproliferation and plaque formation. By binding exclusively to p19, tildrakizumab prevents IL-23 from interacting with its receptor on T cells, innate lymphoid cells, and keratinocytes, thereby halting downstream inflammatory signaling without affecting IL-12 pathways.
Approved by the FDA in 2018, tildrakizumab is indicated for adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Its subcutaneous formulation and extended dosing interval (every 12 weeks) enhance patient convenience compared to more frequent biologic regimens.
Mechanism of action
The core mechanism of tildrakizumab centers on its high-affinity binding to the p19 subunit of IL-23, inhibiting its association with the IL-23 receptor (IL-23R). This blockade occurs at the cellular level in dendritic cells, keratinocytes, and T helper 17 (Th17) cells, preventing activation of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway.
Upon IL-23 receptor engagement, JAK2 and TYK2 phosphorylate STAT3, inducing transcription of RORt —the master regulator of Th17 differentiation. This results in robust secretion of IL-17A/F, IL-22, and TNF-, which amplify keratinocyte proliferation, chemokine production (e.g., CXCL8/IL-8, CCL20), and neutrophil recruitment, perpetuating psoriatic plaques.
- Selective p19 targeting: Spares IL-12 (p40 shared subunit), preserving Th1 immunity against infections.
- Downstream effects: Reduces epidermal hyperplasia, vascular proliferation, and inflammatory infiltrates in lesional skin.
- Gene expression normalization: Lowers IL-19, IL-20, and psoriasis-associated chemokines post-treatment.
Histological studies from phase I/II trials confirm rapid normalization of psoriatic skin architecture, with 67% reduction in severity scores after intravenous dosing, effects sustained subcutaneously.
Psoriasis and the role of IL-23
Plaque psoriasis affects 2-3% of the global population, characterized by well-demarcated erythematous plaques with silvery scales due to dysregulated immune-keratinocyte interactions. Genome-wide association studies implicate IL-23 pathway genes, with elevated p19/p40 expression in lesional dermis versus healthy skin.
IL-23 sustains chronic inflammation by:
- Promoting Th17, Tc17, and ILC3 cytokine release (IL-17/IL-22).
- Inducing keratinocyte antimicrobial peptides (e.g., defensins) and chemokines that recruit neutrophils/mast cells.
- Maintaining epidermal acanthosis and parakeratosis in imiquimod-induced models.
Targeting IL-23p19 interrupts this self-amplifying loop more selectively than p40 inhibitors like ustekinumab, yielding superior long-term skin clearance.
Clinical trials and efficacy
Efficacy was established in two identically designed phase 3 trials: reSURFACE 1 (NCT01729754) and reSURFACE 2 (NCT01729767), involving 1,862 patients with moderate-to-severe plaque psoriasis (PASI 60;12, sPGA 60;3). Patients received subcutaneous tildrakizumab 100 mg, 200 mg, placebo, or etanercept (active comparator in reSURFACE 1).
Co-primary endpoints at week 12: PASI75 (60;75% improvement) and PGA 0/1 (clear/minimal).
| Trial | Tildrakizumab 100 mg | Tildrakizumab 200 mg | Placebo |
|---|---|---|---|
| reSURFACE 1 PASI75 | 62% | 64% | 6% |
| reSURFACE 1 PGA 0/1 | 58% | 62% | 6% |
| reSURFACE 2 PASI75 | 61% | 66% | 9% |
| reSURFACE 2 PGA 0/1 | 57% | 63% | 7% |
All comparisons p<0.0001. Responses deepened over time: PASI90 at week 28 was 70-87% (100/200 mg), PASI100 40-53%. Maintenance through 5 years in extensions showed >80% PASI75 with every-12-week dosing.
Phase II data supported dose selection: 200 mg achieved 74% PASI75 at week 16 vs. 66% for 100 mg. Scalp, nail, and palmoplantar psoriasis also improved significantly.
Dosing regimen
Tildrakizumab is administered subcutaneously under physician supervision. Standard regimen:
- Induction: 100 mg at Week 0 and Week 4.
- Maintenance: 100 mg every 12 weeks thereafter.
For incomplete responders (PASI <50 at week 16), escalate to 200 mg or consider alternatives. No loading dose required; steady-state achieved by week 12. Administer in thighs, abdomen (avoid 5 cm around navel), or upper arms; rotate sites.
Pharmacokinetics
Following subcutaneous injection, tildrakizumab exhibits:
- T_max: 6 days.
- Half-life: 23 days.
- C_max (100 mg): 5-7 μg/mL at steady-state.
- Clearance: Low (0.02-0.04 L/day), independent of body weight, age, or sex.
No dose adjustments for renal/hepatic impairment or mild-moderate obesity. Pediatric pharmacokinetics unestablished.
Side effects and safety
Tildrakizumab demonstrates a favorable safety profile, with adverse events (AEs) comparable to placebo through 5 years.
| Adverse Event | Incidence (100 mg, up to week 64) |
|---|---|
| Upper respiratory infection | 14-19% |
| Injection site reaction | 3% |
| Headache | 5% |
| Serious AE | 3% (similar to placebo) |
Infections: No increased risk of serious infections (0.6/100 patient-years). Screen for latent TB/hepatitis B prior to initiation.
Malignancy: Standardized incidence ratio 0.72 (95% CI 0.36-1.29) over 5 years.
Contraindications: Hypersensitivity to tildrakizumab. Use caution in IBD history due to IL-23 role in gut homeostasis.
Interactions and contraindications
- Vaccinations: Avoid live vaccines; update non-live prior.
- CYP450 substrates: Minimal impact (no significant IL-6 inhibition).
- Immunosuppressants: Limited data; monitor closely.
Contraindicated in active serious infections or hypersensitivity.
Pregnancy and breastfeeding
Limited human data (Category not assigned). Animal studies show no adverse effects at 89x human exposure. IgG crosses placenta; consider risks/benefits. Unknown in breast milk; pump/discard during treatment.
Monitoring
- Baseline: CBC, LFTs, TB test, hepatitis serology.
- Ongoing: Signs of infection; annual skin cancer exam.
- Response: PASI/sPGA at weeks 12/16; adjust if inadequate.
Frequently Asked Questions
What is the success rate of tildrakizumab?
Approximately 62% achieve PASI75 and 58% PGA 0/1 at week 12; >80% maintain long-term with every-12-week dosing.
Does tildrakizumab cure psoriasis?
No, it controls symptoms by targeting IL-23; plaques may recur upon discontinuation, though responses persist 20+ weeks post-treatment.
Is tildrakizumab safe for long-term use?
Yes, 5-year data show sustained efficacy/safety, low immunogenicity (5% antibodies, no neutralization impact).
Can tildrakizumab be used with other psoriasis treatments?
Combination data limited; not recommended with systemic immunosuppressants due to infection risk.
How soon does tildrakizumab work?
Visible improvement by week 4; peak response week 12-28.
References
- Long-Term Efficacy of Tildrakizumab in the Treatment of Psoriasis 20 ) — Skin Therapy Letter. 2023. https://www.skintherapyletter.com/psoriasis/tildrakizumab/
- Tildrakizumab – DermNet — DermNet NZ. 2024-01-15. https://dermnetnz.org/topics/tildrakizumab
- A Review of the Efficacy and Safety for Biologic Agents Targeting IL-23 in Psoriasis — Frontiers in Medicine. 2021-09-03. https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2021.702776/full
- Tildrakizumab: Uses, Interactions, Mechanism of Action — DrugBank. 2025. https://go.drugbank.com/drugs/DB14004
- Ilumya (Tildrakizumab-asmn), Interleukin-23 Antagonist — American Health & Drug Benefits. 2019-03-01. https://www.ahdbonline.com/issues/2019/march-2019-vol-12-tenth-annual-payers-guide/ilumya-tildrakizumab-asmn-interleukin-23-antagonist-approved-for-the-treatment-of-patients-with-moderate-to-severe-plaque-psoriasis
- Tildrakizumab: A Review in Moderate-to-Severe Plaque Psoriasis — PubMed (Drugs Journal). 2019-04-01. https://pubmed.ncbi.nlm.nih.gov/30924030/
Read full bio of medha deb